When Olivier Vanpé joined CEFALY Technology (then called STX-Med) in 2010, the company was working on multiple projects and products. One in particular caught Olivier’s attention: a non-drug migraine treatment device.
Olivier didn’t live with migraine himself. But he understood its impact on patients’ lives, because he had relatives with migraine—including one who lived with severe chronic migraine.
“CEFALY was a dynamic small startup on a mission to improve people’s lives with non-drug, innovative solutions,” Olivier said. Was it truly possible for patients to find relief from migraine without the side effects of medication? Olivier and the CEFALY team were determined to find out.
The early years of CEFALY
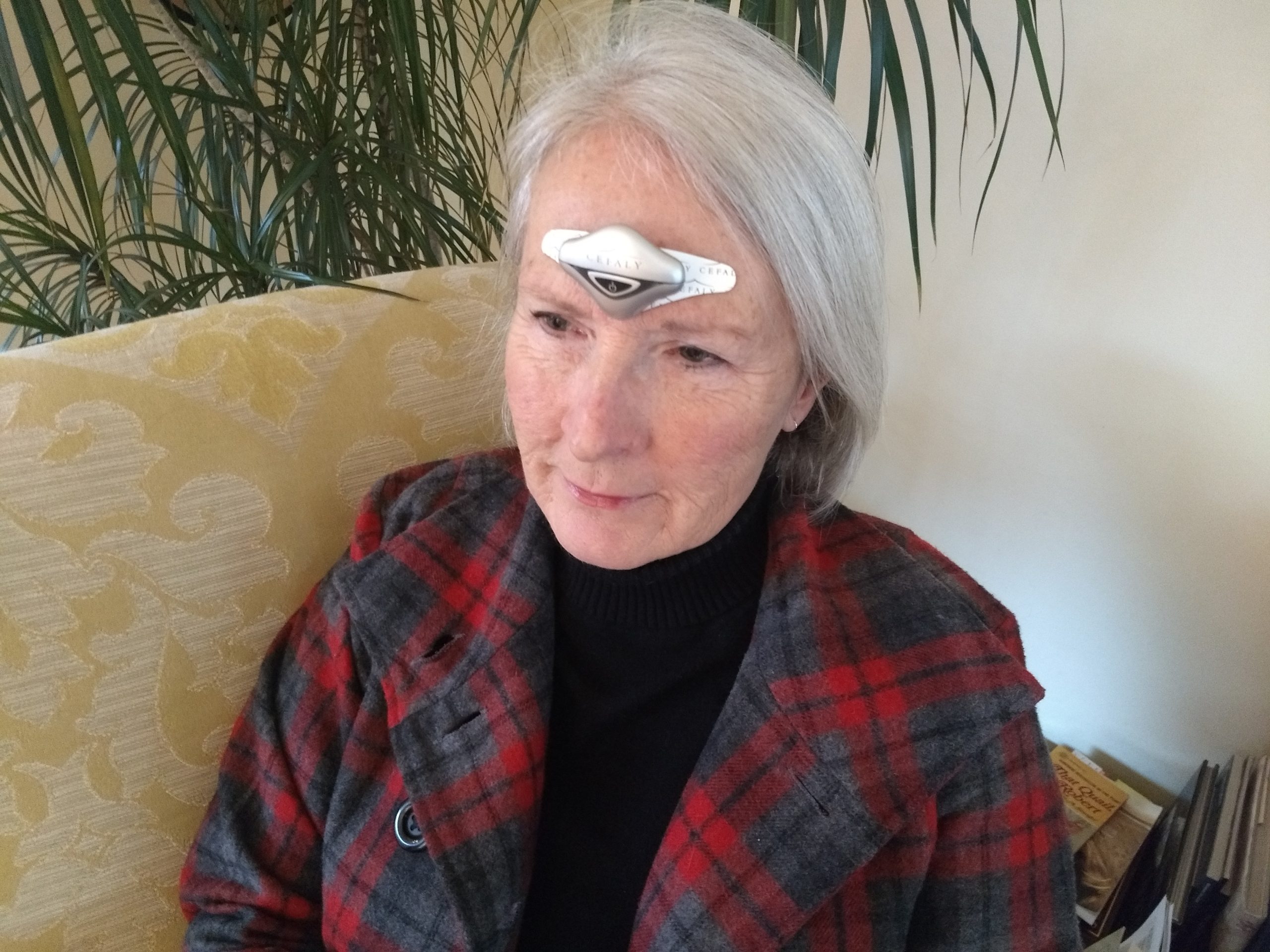
When Olivier joined the company, it was just beginning to market the first version of the CEFALY device: a headband-style device that used non-invasive neuromodulation to prevent migraine.
CEFALY was still a young company, founded in 2004 in Belgium. It faced many challenges on the road to growth. Belgium was a small country with complex regulations. The company would have to rapidly expand its international reach in order to gain enough traction to survive.
Research into CEFALY’s efficacy was promising, but still ongoing. And the company itself was tiny. Seven people did everything, including Olivier and the two founders.
“Everyone was on deck, rowing as needed,” Olivier said. He remembers taking CEFALY to an enormous tradeshow in Germany. As Olivier helped people try the device, countless patients told him about their experience living with the debilitating symptoms of migraine. They told him about all the treatments they had tried—and all the treatments that had failed.
Olivier realized how important CEFALY’s work was to these patients. They had to succeed.
CEFALY Technology: A pioneer in neuromodulation for migraine
Migraine is one of the world’s most common neurological disorders, affecting 1.16 billion people worldwide. It’s also one of the most common causes of disability for children, adolescents, and adults (particularly women) in their working years.
There is no cure for migraine. It’s a complex condition that can look very different in one patient vs. another. And even though migraine occurs in so many people, science does not yet fully understand what causes it. While the world has seen many new pharmaceutical treatment options in the last two decades, migraine medications can have serious side effects and don’t work well for everyone.
Around the early 2000s, researchers began to recognize the potential of neuromodulation to treat migraine. Neuromodulation involves targeting specific nerves to change their behavior.
So what is the CEFALY device, and how does it treat migraine? CEFALY uses a specific type of neuromodulation called external trigeminal nerve stimulation (e-TNS). The trigeminal nerve is the largest cranial (head) nerve and the primary pathway for migraine pain signals.
Worn on the forehead, CEFALY sends tiny, precise electrical pulses through an electrode to stimulate and desensitize this nerve. Early studies had shown that this gentle stimulation could both relieve an active migraine attack and reduce the frequency of migraine attacks. Best of all, studies showed that CEFALY was safe to use, with no harmful side effects.
As the CEFALY Technology team continued to refine the device and study the results, they never lost sight of their mission: helping migraine patients live better lives.
Olivier remembers one woman who called CEFALY in tears. Her daughter’s chronic migraine was so debilitating, the pain had forced her to quit school and stay in bed several times a week. By using CEFALY, her daughter was able to return to school. “You saved my daughter’s life,” she said. “She has a future now.”
FDA clearance paves the way for CEFALY’s sale in the U.S.
In 2013, the first major, peer-reviewed study of CEFALY as a migraine prevention treatment was published. The Migraine Prevention with a Supraorbital Transcutaneous Stimulator (PREMICE) study demonstrated that with consistent use of the PREVENT mode, CEFALY reduced migraine frequency and helped patients take less acute migraine medication.
In 2014, CEFALY was the first neuromodulation device for migraine to receive FDA clearance for the prevention of episodic migraine. This was a monumental step. FDA clearance meant that the Food and Drug Administration had reviewed the safety and efficacy of CEFALY and authorized its sale in the U.S.
The company doubled in size “almost overnight,” Olivier said. CEFALY Technology opened a U.S. office in Darien, Connecticut. There was only one customer service representative, so everyone pitched in to answer calls. (Today, we have a larger customer service team—still based in Darien! Here’s how to contact CEFALY customer service.)
The evolution of the CEFALY migraine treatment device
Over the next decade-plus, research, patient feedback, and technology innovations helped refine the CEFALY device.
- 2016: CEFALY 2—the first CEFALY to adhere to an electrode on the forehead—launched in Europe. The same year, CEFALY PREVENT launched in the U.S.
- 2017: CEFALY was FDA-cleared for the acute treatment of migraine.
- 2018: The company introduced the CEFALY DUAL, which combined ACUTE and PREVENT treatment modes in a single device. Also, the Acute Migraine Therapy with External Trigeminal Neurostimulation (ACME) study was published. This study showed that one hour of ACUTE treatment with CEFALY resulted in significant headache pain relief and was well tolerated.
- 2020: CEFALY marked a major milestone. It was approved for over-the-counter use, making it the first FDA-cleared device used for the treatment and prevention of migraine available without a prescription.
- 2021: The CEFALY Enhanced was launched with wireless charging, a longer battery life and a new, intuitive design. CEFALY also introduced the CeCe Migraine Management app: a simple, intuitive way for users to track migraine triggers and symptoms.
- 2022: A new model was introduced in the U.S. and Canada. The Bluetooth-enabled CEFALY Connected paired with the CeCe app to help users track their treatment sessions and gain new insights into their journey toward migraine relief. That same year, the CEFALY Coach program began offering personalized assistance to customers in the U.S. and Canada with a free, one-on-one session with a CEFALY expert.
- 2022: The TEAM (Trial of e-TNS for the Acute treatment of Migraine) study, CEFALY’s largest study to date, demonstrated that ACUTE treatment not only relieves or stops migraine pain but also relieves most bothersome migraine symptoms, such as vomiting, sound sensitivity, and light sensitivity.
- 2023: CEFALY Enhanced became available in Europe. CEFALY unveiled Headaches.com, the source for unbiased, medically reviewed information on neuromodulation for migraine.
- 2024: CEFALY Technology marked 20 years of migraine treatment innovation. An update to the CeCe app enabled two-way communication with CEFALY Connected, so that patients could use CeCe to select, start, stop, and adjust their CEFALY treatments.
- 2024: CEFALY Connected became the #1 neuromodulation device for migraine among veterans served by the Veterans Health Administration. Also, the CEFALY Pregnancy Registry launched to gather information about the safety of external trigeminal nerve stimulation (eTNS) as a treatment for migraine during pregnancy.
- 2025: CEFALY’s migraine innovations continued with new research, new education initiatives, and new updates to the CeCe app, making it more effective and easier to use with CEFALY Connected.
Now General Manager Belgium & Analytics for CEFALY, Olivier is helping guide CEFALY Technology into the next phase of its growth. The mission remains the same: To provide innovative, ever-evolving technology that enables people with migraine to take control of their treatment and live happier, healthier lives.
Get Drug-Free Migraine Relief With CEFALY
Shop Now
90-day money back guarantee
FDA-cleared
financing available