“We are excited to start the New Year on a new path,” said Jennifer Trainor-McDermott, CEO with CEFALY Technology. “We are developing very focused initiatives to help migraineurs across Canada access relief and more quickly. This strategic move to relocate distribution in-house will provide more flexibility to meet our growing demand.”
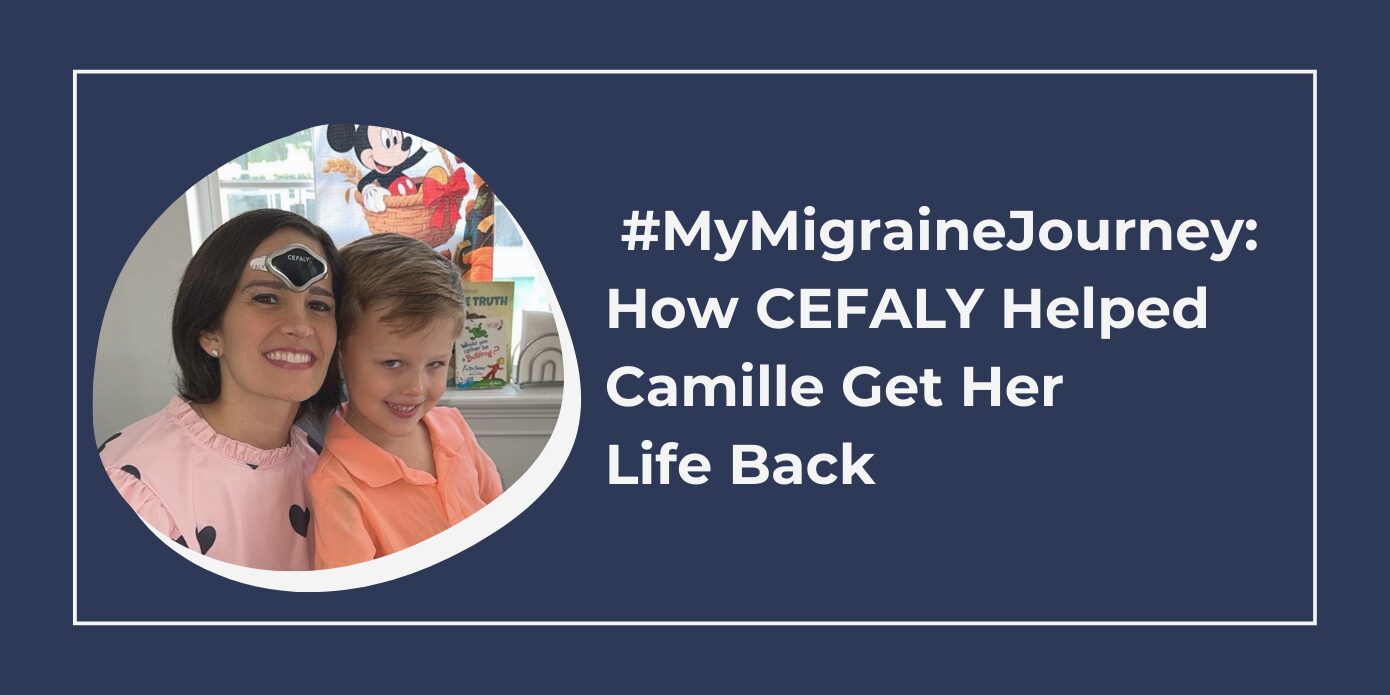
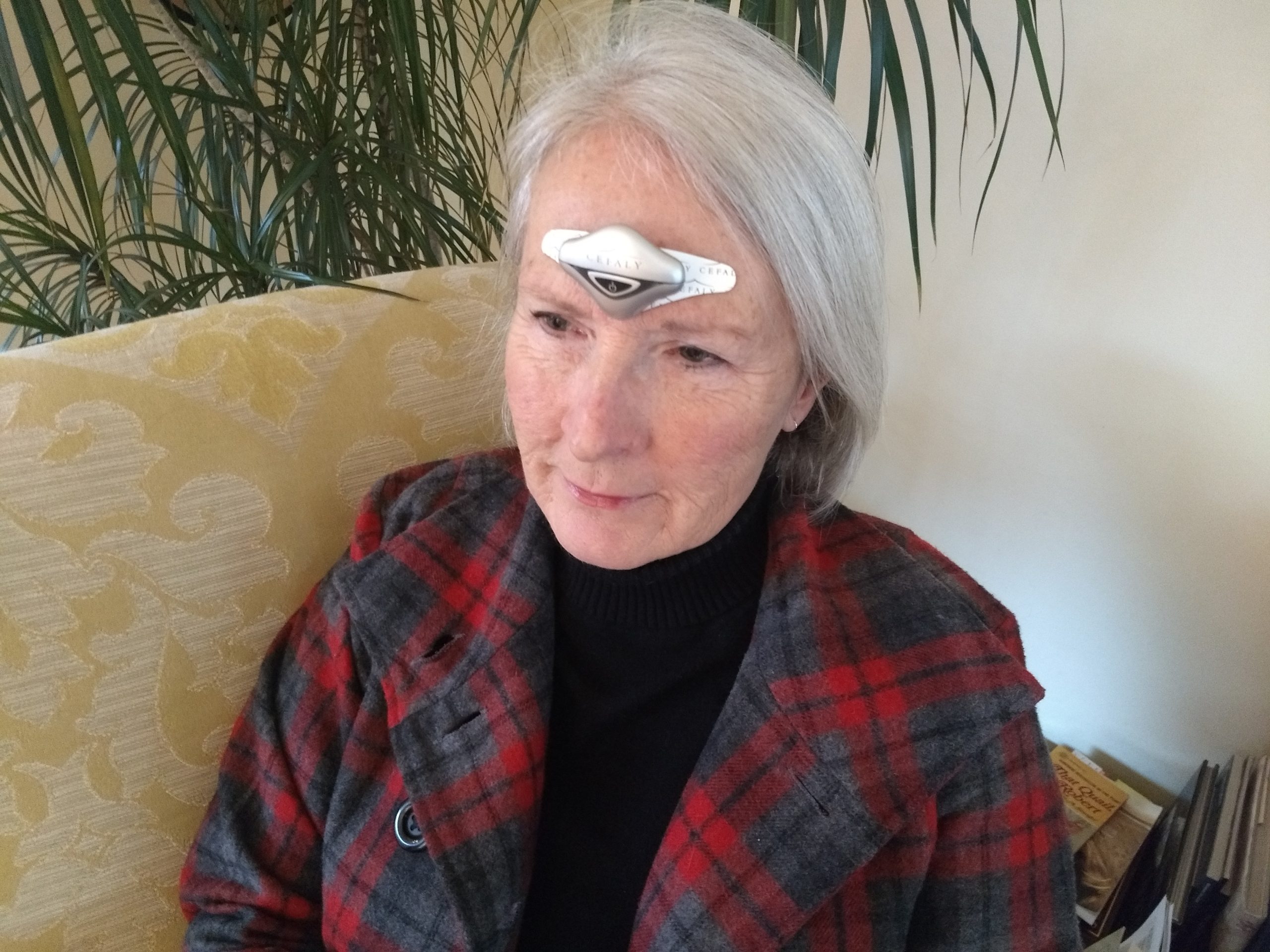
CEFALY has rapidly become a global brand, widely recognized among the migraine community, which is estimated to be around 1 billion worldwide1. The CEFALY device is the first FDA Cleared/CE Marked medical technology of its kind for the treatment of migraine headaches. It is a non-invasive device placed on the forehead that uses two distinct programs (ACUTE and PREVENT) to stimulate and desensitize the area research identifies as a center for migraine pain, the Trigeminal nerve. Since CEFALY is not a drug, it can be used as a standalone option or with an existing treatment. Patients who use the device consistently show a decrease in migraine days and in intake of migraine medication.
In addition to gaining access to CEFALY’s latest technology, consumers in Canada will experience the company’s newly refreshed brand and web presence: https://www.cefaly.com/en-ca
About CEFALY Technology
CEFALY Technology is a Belgium-based company, with US offices based in Wilton, Connecticut, specializing in electronics for medical applications. It has developed external cranial stimulation technology for applications in the field of neurology; in particular for treating migraines.
Try CEFALY for 90 days, and if you are not satisfied with the product, send it back for a full refund. We’ll even email you a PrePaid FedEx shipping label, so you don’t have to pay for the return shipping.
For more information about CEFALY Technology visit: https://www.cefaly.us.
For more information on purchasing CEFALY in Canada visit: https://www.cefaly.com/en-ca.
Sources
1 Migraine facts. American Migraine Foundation website. Accessed January 13, 2020